On this page, a simple overview of the DEBkiss physiological model is presented. This summary is highly simplified and more detailed information is available in the scientific literature (Jager, 2020; Jager et al., 2013).
The DEBkiss model describes the assimilation of energy from food and its subsequent allocation to various organismal process. Assimilates from food are initially divided into two branches. The first, the somatic branch, includes energy for maintenance of existing body mass (structure) and the growth of new structure. The proportion of available assimilates allocated to the somatic branch is denoted by the Greek letter kappa (κ). The other branch, the reproductive branch, includes the energy allocation to sexual maturity and reproduction. The fraction allocated to the reproductive branch is denoted (1- κ). The model is depicted in Figure 1 below.
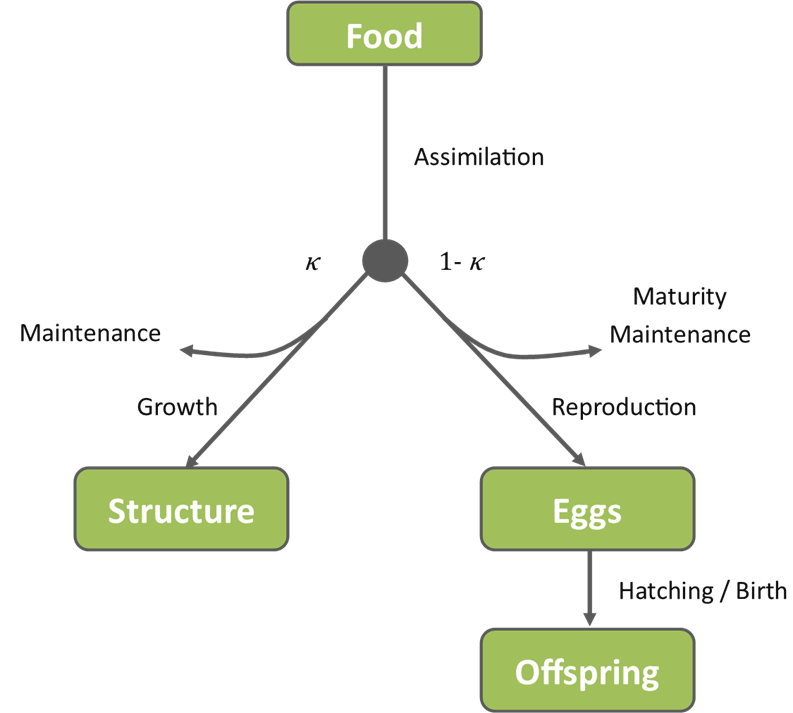
Figure 1: Schematic showing the allocation of assimilates from food in a sexually mature female in the DEBkiss model. For juveniles, the 1-kappa branch is not explicitly followed as there is no reproduction. Instead, the allocated energy is assumed to be used for maturation.
Somatic maintenance and maturity maintenance are essential processes, for survival and maintaining sexual maturity, respectively. As such, they take priority over the processes of growth and reproduction if resources become limited. Additionally, reproduction begins at a critical body size and reproductive rate increases with body size thereafter. The ultimate reproductive endpoint is the cumulative number of offspring. For some species this may be counted as the number of eggs while for others, offspring are counted after hatching. The cumulative number of hatched offspring depends on both the mother’s egg production and survival rates to hatching/birth.
Stress on physiological processes
Exposure to a chemical leads to the accrual of internal ‘damage’ which is also repaired at a certain rate. Under constant exposure, the rates of damage accrual and repair reach equilibrium, so that the overall damage level also remains constant. Damage leads to ‘stress’ which can be applied to one or more processes in the physiological model. The various processes are referred to as ‘physiological modes of action’ (pMoAs) and each may affect the rates of growth and/or reproduction:
- Stress on assimilation (Figure 2A) reduces the total energy available. Maintenance rates are unaffected, so the rates of growth and reproduction are both reduced. Maximum body size is also reduced.
- Stress on maintenance (Figure 2B) results in increased somatic maintenance and maturity maintenance (which is calculated as a function of somatic maintenance). As a result, less energy is available for growth and reproduction. Maximum body size is reduced.
- Stress on growth (Figure 2C) makes growth a more energetically expensive process and results in reduced growth rate. However, maximum body size is unaffected. There are no effects on reproductive rate at a given body size. However, as reproduction is linked to body size, cumulative reproduction over time will also decrease.
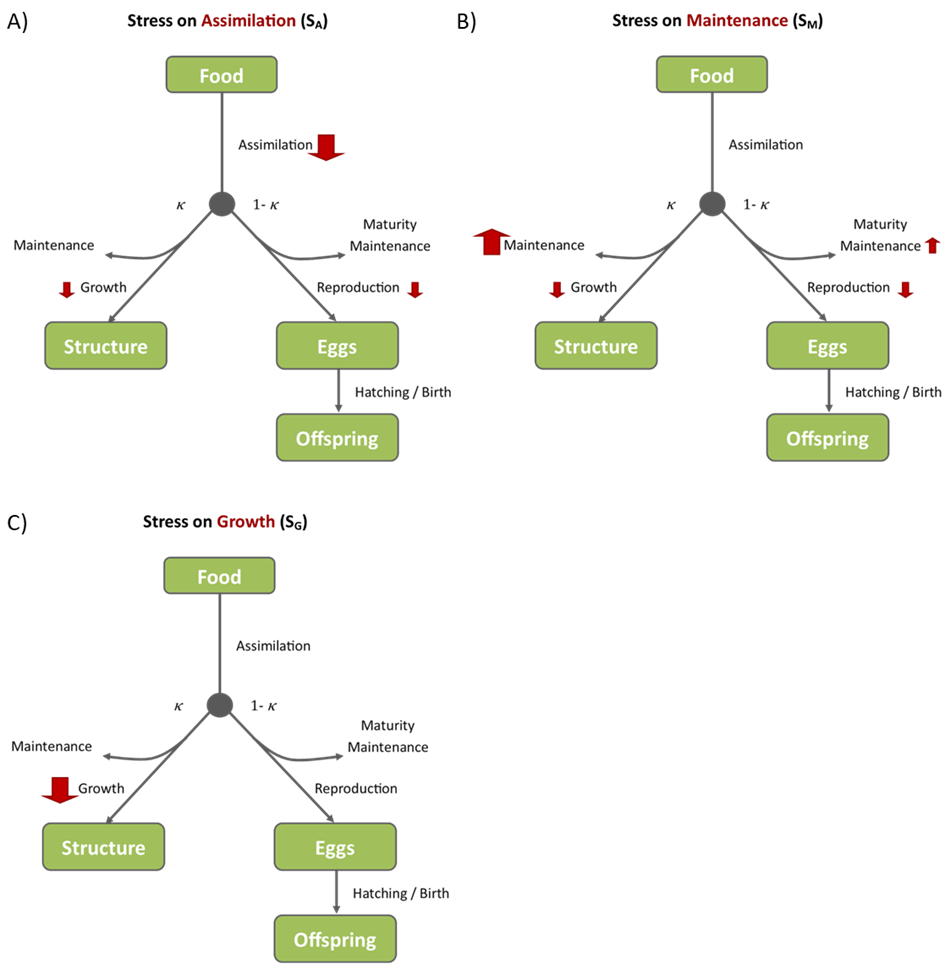
Figure 2: Illustration of the impacts of stress on assimilation (A), maintenance (B), and growth (C). The direct impact of the stress in each pMoA is shown by a large red arrow while any indirect impacts on other processes are shown by smaller red arrows.
Stress may also directly affect reproductive processes:
- Stress on reproduction (Figure 3A) makes the production of eggs a more expensive process, leading to reduced reproduction with no impact on growth processes whatsoever.
- Stress on egg formation or embryo survival (Figure 3B) leads to decreased viable egg production and/or hatching/birth rates. This results in reduced reproductive output in terms of offspring without any effects on growth.
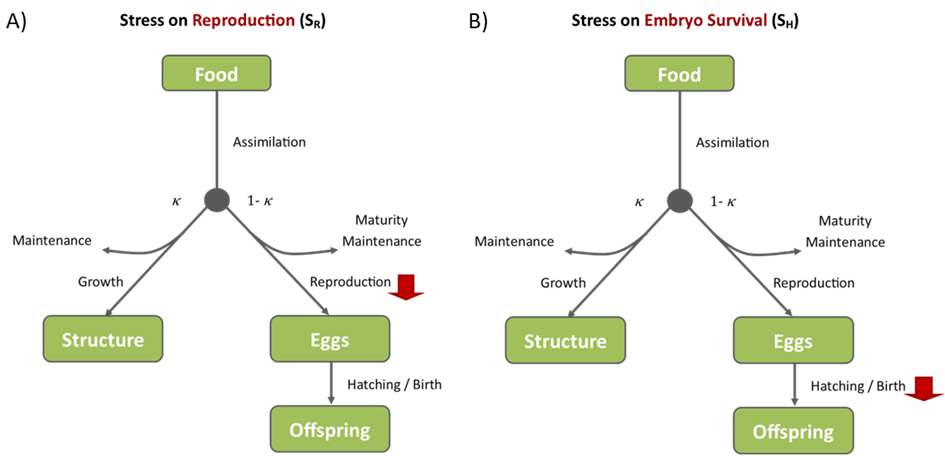
Figure 3: Illustration of the impacts of stress on reproduction(A), and embryo survival (B). The direct impact of the stress in each pMoA is shown by a large red arrow while any indirect impacts on other processes are shown by smaller red arrows.
References
- Jager, T., 2020. Revisiting simplified DEBtox models for analysing ecotoxicity data. Ecol. Model. 416, 108904. https://doi.org/10.1016/j.ecolmodel.2019.108904
- Jager, T., Martin, B.T., Zimmer, E.I., 2013. DEBkiss or the quest for the simplest generic model of animal life history. J. Theor. Biol. 328, 9–18. https://doi.org/10.1016/j.jtbi.2013.03.011