Mechanistic effect models (MEMs) have great potential for use in environmental risk assessment (ERA) of chemicals such as pesticides. For this purpose, toxicokinetic-toxicodynamic (TKTD) models have received particular attention. TKTD models based on dynamic energy budget theory (DEB-TKTD or DEBtox) simulate sublethal effects, exploring the effects of toxicants on growth and reproduction over time, so are well suited to predict the impacts of realistic, medium & long-term exposure.
In 2018, EFSA scientific opinion on TKTD models for aquatic organisms (EFSA PPR 2018) recognised the great potential of DEB-TKTD models for use in ERA for pesticides. However, it also concluded that these models are not yet ready for use, with the lack of user-friendly DEB-TKTD modelling tools being cited as a major factor.
For this reason, DeEP (DEB-TKTD EPx Predictor) was developed. DeEP provides a user-friendly platform to make predictions relevant to pesticide ERA (specifically, the factor by which realistic exposure would need to be multiplied to cause an X% reduction in body weight or reproduction). This allows non-specialists to extrapolate from laboratory data to realistic field scenarios with DEB-TKTD modelling (specifically the DEBtox2019 implementation; Jager, 2020).
What is DEB-TKTD?
DEB stands for dynamic energy budget and TKTD stands for toxicokinetic-toxicodynamic.
The original DEB model was produced by Bas Kooijman to simulate the acquisition and allocation of energy by organisms as they grow and reproduce (Kooijman, 2000). The simplified DEBkiss (keep it simple, stupid) model was later produced by Tjalling Jager (2013). DEB-TKTD models additionally simulate the uptake and elimination of a compound over time (toxicokinetics) and translate that into stress (toxicodynamics) on DEB model parameters (Jager, 2019) . This allows the effects of untested exposure profiles on endpoints such as growth and reproduction to be predicted.
More recently, DEBtox2019 was published in a study by Tjalling Jager (2020). DEBtox2019 is a model implementation which presents the simplified DEBkiss model with the fewest possible parameters. This makes it the most accessible and user-friendly DEB-TKTD implementation to date and is the framework used by this software.
Reduced toxicokinetic model
In the reduced TK model, toxicokinetics is combined with damage dynamics into a single compartment. This compartment has first-order kinetics, determined by a 'dominant' rate constant kd. The scaled damage is subsequently linked to stress on growth and reproduction.
Brief Description of the DEBkiss model
In the following the DEBkiss model is described briefly. You can find a more detailed description here.
The DEBkiss model simulates the organismal processes of assimilation from food, somatic maintenance, growth, and reproduction (Figure 1). A constant fraction of assimilates, denoted κ (kappa), is allocated to maintenance and growth. As the organism grows, maintenance costs increase, and so less energy is available for growth. Maximum size is reached when all assimilates from the kappa fraction are required for maintenance and growth ceases.
The other fraction of assimilates, given by 1-κ (kappa), is allocated to maturity until puberty and then to reproduction. Maturity is not followed as a state variable, instead puberty (the start of reproductive investment) occurs at a fixed body length. Reproduction is modelled at a constant rate, rather than in discrete reproduction events (as occur in reality). This rate increases as the organism grows and so also reaches its maximum along with body size.
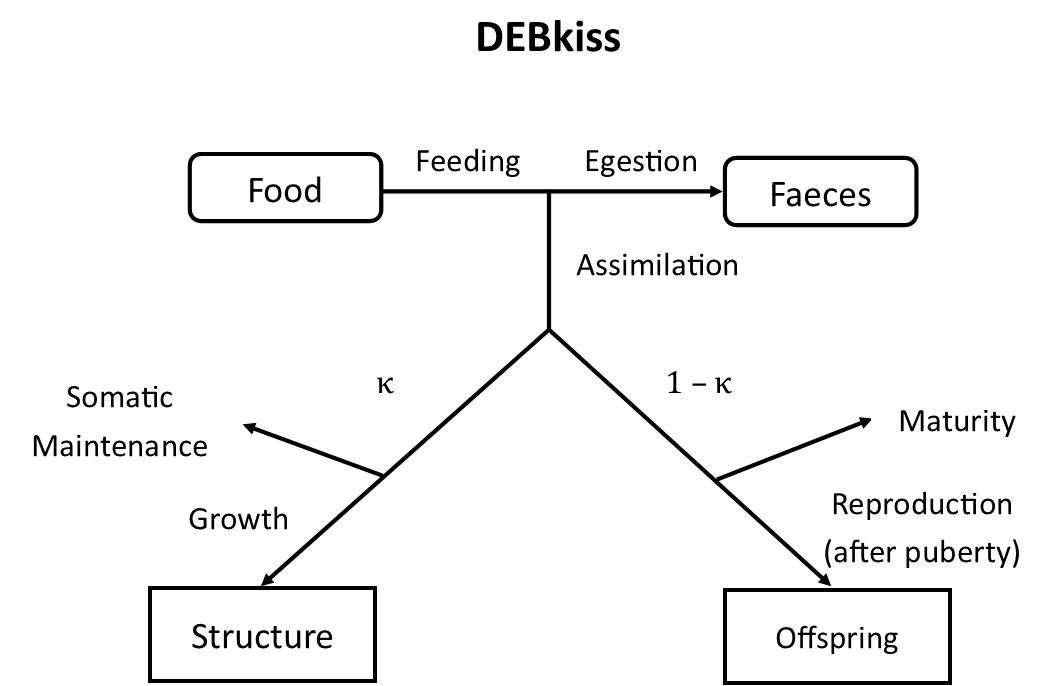
Figure 1: Schematic showing the processes modelled in the DEBkiss model. An organism assimilates resources from food which are then allocated to maintenance, growth, maturation or reproduction.
Brief Description of the DEB-TKTD model
When an organism is exposed to a chemical compound it is assumed to accrue ‘scaled damage’ over time. This damage is repaired when exposure decreases or ceases. If the accrued damage exceeds a threshold level, it places stress on biological processes - leading to effects on growth and/or reproduction. Stress may be applied to one or several parameters within the DEBkiss model. Whether a compound affects assimilation, maintenance, growth, or reproduction is known as its physiological mode of action or pMoA.
Moving time window and EPx
The software iterates over a user provided exposure profile using a ‘moving time window’ approach (Sherborne et al., 2020). This means that predictions are made for multiple overlapping windows within the profile, moving forward at a user-specified step length. The software finds the factor by which the exposure profile (external concentration time series) in each window would need to be multiplied in order to cause a X% reduction in growth or reproduction. The lowest EPx for any time window determines whether criteria for risk assessment are met.
References
- Jager, T. (2019). Making sense of chemical stress. Application of Dynamic Energy Budget theory in ecotoxicology and stress ecology (2.0). leanpub
- Jager, T. (2020). Revisiting simplified DEBtox models for analysing ecotoxicity data. Ecological Modelling, 416, 108904. https://doi.org/10.1016/j.ecolmodel.2019.108904
- Jager, T., Martin, B.T., & Zimmer, E.I. (2013). DEBkiss or the quest for the simplest generic model of animal life history. Journal of Theoretical Biology, 328, 9–18. https://doi.org/10.1016/j.jtbi.2013.03.011
- Kooijman, S.A.L.M. (2000). Dynamic Energy and Mass Budgets in Biological Systems (2nd ed.). Cambridge University Press. https://doi.org/10.1017/CBO9780511565403
- Sherborne, N., Galic, N., & Ashauer, R. (2020). Sublethal effect modelling for environmental risk assessment of chemicals: Problem definition, model variants, application and challenges. Science of The Total Environment, 745, 141027. https://doi.org/10.1016/j.scitotenv.2020.141027